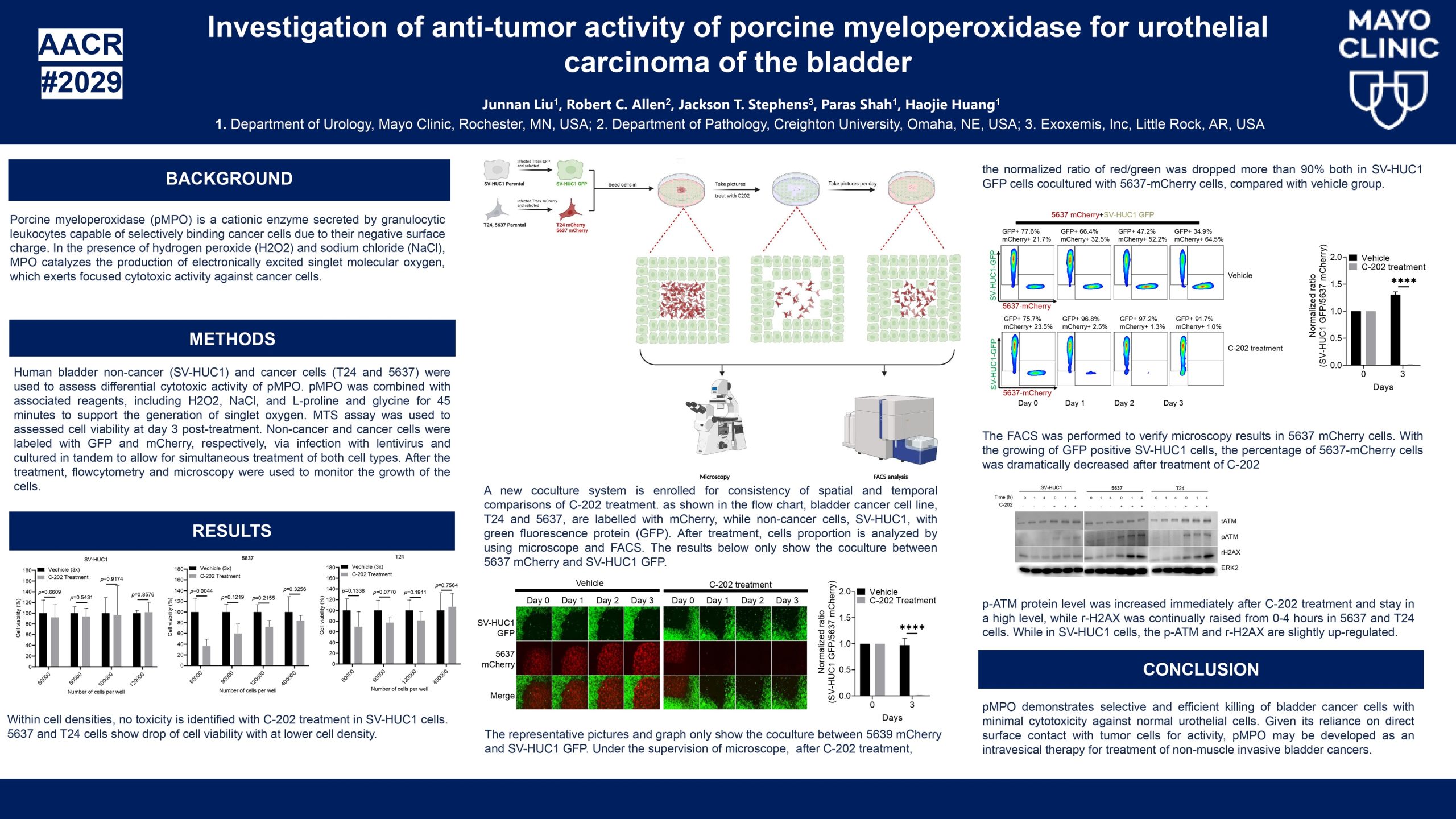
Human Clinical Trials
Exoxemis has completed a placebo controlled, randomized, international, Pivotal, Phase III, human clinical trial with 250 human subjects in each arm, at 25 U.S. and 5 Israeli sites. This study provides direct evidence that Zempia® is safe for use in open wounds—it does not interfere with normal wound healing.
About Zempia®
Action of Zempia®
Has broad microbicidal activity against all wound pathogens; kills antibiotic resistant bacteria; is effective in killing gram-negative and gram-positive bacteria, viruses, spores, yeast and fungi.
E-101 Solution (Zempia®) Demonstrates Viricidal
Properties Against Coronavirus in Mucin.
ePoster with audio and slide presentation from the
The ESCMID Conference on Coronavirus Disease (ECCVID)
September 2020
For Immediate Release
Groundbreaking Research Points Towards Zempia®
As a Proposed New Weapon in Medicine’s Armamentarium to Fight Sepsis
Myeloperoxidase and Eosinophil Peroxidase Conclusively
Shown to Inactivate Endotoxin and Lipid A.
Jackson T. “Steve” Stephens, Founder and CEO of Exoxemis, Inc. (www.exoxemis.com) announced the ublication of a peer reviewed article, which he co-authored with Dr. Robert C. Allen just published in the Journal of Immunology Research titled Myeloperoxidase and Eosinophil Peroxidase Inhibit Endotoxin Activity and Increase Mouse Survival in a Lipopolysaccharide Lethal Dose 90% Model. Full article text available here.
This is a ground breaking study relative to the further investigation and testing of Zempia®, our trade name for myeloperoxidase (MPO), to combat sepsis,” commented Mr. Stephens. “Our study utilized the Limulus amebocyte lysate (LAL) test which is the definitive test recognized by the FDA with regard to detecting endotoxins. Additionally, the mouse lethal dose 90% (LD90) survival model testing provided ‘unquestionably significant’ in-vivo data that demonstrated MPO increased survival in mice administered MPO with an otherwise lethal dose of endotoxin.”
Antibiotics and antiseptics can kill bacteria but they can leave behind toxins (poisons). In the case of Gram-negative bacteria release of residual lipopolysaccharide results in potentially lethal endotoxemia. “Our extensive in-vivo and in-vitro testing over the last twenty-seven years has given us direct evidence that Myeloperoxidase (MPO) when mixed with a
substrate solution containing Glucose Oxidase plus glucose produces hypochlorous acid and singlet oxygen which, in addition to killing pathogens, inactivates endotoxins from gram negative bacteria and other toxins from gram-positive bacteria. This new study (just released) demonstrates that MPO alone (in the absence of hypochlorite and singlet oxygen
Production) provides nonenzymatic protection against endotoxin, i.e., lipopolysaccharide (LPS) as well as its toxic lipid A component. This is a huge discovery relative to combatting gram negative sepsis.”
According to the CDC (Center for Disease Control and Prevention) “at least 1.7 million adults in America develop sepsis.Nearly 270,000 Americans die as a result of sepsis. 1 in 3 patients who die ina hospital have sepsis” The National Institutes of Health estimates that more people die from sepsis than from prostate cancer, breast cancer and AIDS combined.
Exoxemis is a biomedical research company focused on the research and development of Zempia®, the company’s trade name for Myeloperoxidase. “Exoxemis is the only biomedical research company in the world that has been able to make MPO in large, pure quantities at a price that makes it suitable for commercial use either in conjunction with a medical
device or as a therapeutic,” said Mr. Stephens.
Exoxemis has completed three Phase | and one Phase III human clinical trial with 250 human subjects in each arm, at 25 U.S. and 5 Israeli sites, A primary safety end point of the Phase Ill study was to compare Zempia® (referred to as E-101 in the study) to saline. The safety conclusions of this study in part conclude: “This E-101 Solution-related safety profile
demonstrates no increased documentation of abnormal laboratory tests (i.e., serum chemistry, hematological, urinalysis or coagulation test), physical examination (primary intent wound healing followed for up to six months after surgery), quality of life assessments, tolerability or serious event reporting among E-101 Solution treated subjects compared to saint ih
this protocol.” The Phase III Clinical Report Summary (CRS) is on file with the FDA and is available to interested parties.
The prior in vitro, efficacy studies—laboratory and animal testing—have generated direct evidence that Zempia®:
- Has broad microbicidal activity against all wound pathogens; kills antibiotic resistant bacteria; is effective in killing gram-negative and gram-positive bacteria, viruses, and spores, yeast, and fungi.
- Does not harm tissue or blood cells because it selects for, and binds to, pathogens.
- Does not cause local irritation or sensitization
- Is not systemically absorbed (thereby eliminating systemic toxicity)
- Has microbicidal activity in wound environments with blood and/or exudate where most other antiseptics in use today (except for silver) are inactivated by miniscule amounts of blood. Silver, however, is a small ion and can be absorbed—hence has potential toxic side effects although no evidentiary safety or efficacy studies on silver or any other antiseptic in use today have been conducted and are on file at FDA.
- Inactivates endotoxin and its highly toxic component Lipid A. Other antiseptics kill bacteria but they can leave the endotoxins that cause sepsis, Zempia is a breakthrough in preventing sepsis.
Exoxemis holds a number of method and composition Patents throughout the world, and a number of patents are currently pending. In addition, the company has registered Zempia® and has pending trademarks throughout the world.
An exclusive licensing agreement is available for Zempia® for further Study and testing with applications in a number of potential markets: for use in preventing sepsis, in conjunction with large joint implants; urinary catheters; dental implants and oral surgeries; for use in the ER, in wound treatments, colorectal, HEENT, Plastics/Burn-really in any surgery or
condition involving an open wound or normal flora environment that is susceptible to infection. This includes skin care, dermatology, even cosmetics.
“We are also interested in pursuing further investigation and testing with regard to the use of Zempia® to counter biological warfare. Our tests show that Zempia® has broad antimicrobial action against bacteria that medics and first responders might not normally encounter—to help protect them while administering medical treatment and to contain the biological threat when every second counts,” said Mr. Stephens.
Further research and test data on Zempia®, E-101 and MPO is available by visiting the Exoxemis website: www.exoxemis.com

Jackson T. “Steve” Stephens
Founder & CEO
PARTNER WITH EXOXEMIS
Today, Exoxemis, Inc. is well qualified to partner with a medical device or pharmaceutical company to conduct further testing, obtain regulatory approval and to more quickly optimize Zempia® market share. An exclusive licensing agreement is available for Zempia® for several applications in a number of potential markets: for use in conjunction with large joint implants; urinary catheters; dental implants and oral surgeries; for use in the ER, in wound treatments, colorectal, HEENT, Plastics/Burn. . . .really in any surgery or condition involving an open wound that is susceptible to infection. This includes skin care, dermatology, even cosmetics. We believe that Zempia® could be an effective weapon to fight AMR and HAI’s infections that are now a crisis and plaguing our hospital systems.
“We’re still in the age of the telegraph with regard to topical antiseptic R&D. While further testing and approval for Zempia® is necessary, we’re very excited about it’s many commercial applications.”
Jackson T. “Steve” Stephens
Founder & CEO
In 1919, Alexander Fleming proved that carbolic acid and other antiseptics indiscriminately kill pathogens as well as healthy tissue including the white blood cells that offer the body’s own defense mechanism against infection Researchers in the field of antiseptics have made futile attempts to balance pathogenic, germ cell killing with the sparing of human cells. Adding to the problem, it was also discovered that antiseptic germ-killing properties are inactivated in small amounts of human blood but still kill human cells.
The unsatisfactory problem with antiseptics was ignored for decades primarily due to the effective use of antibiotics. After decades of over-prescribing antibiotics and the dilution of antiseptics, germs have built up a resistance to both topical and systemic agents meant to kill them.